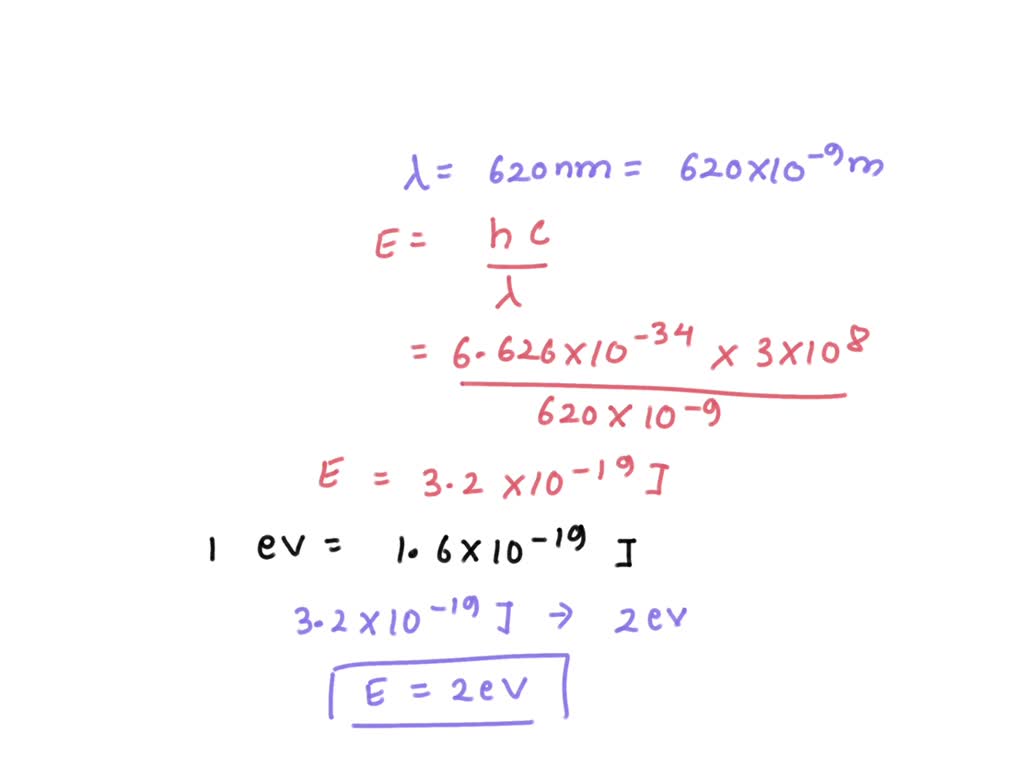
SOLVED: What is the kinetic energy of an electron ejected from a sodium surface whose work function (binding energy) is 2.3 eV when illuminated by red light of wavelength 620 nm?
kinetic energy of an electron which is associated with de Broglie's wavelength 20 angstrom is 1)1.0eV, 2)1.51eV, 3)0.59eV, 4)0.38eV.

Total energy E, kinetic energy T , single electron energy E , Coulomb... | Download Scientific Diagram
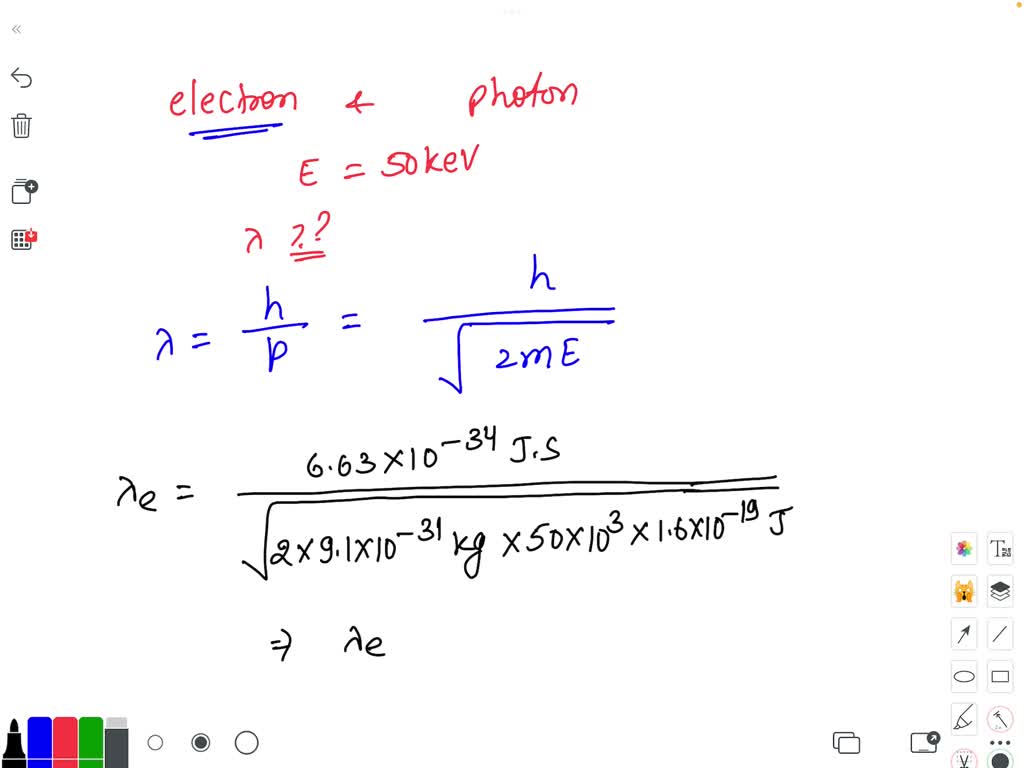
SOLVED: An electron and a photon each have kinetic energy equal to 50 keV. What are their de Broglie wavelengths?

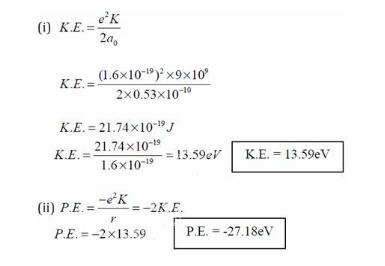
The Kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is: (a 0 is Bohr radius)

If an electron and a proton have the same kinetic energy,the ratio of their de Brogile wavelengths will be - Physics - Dual Nature Of Radiation And Matter - 14107279 | Meritnation.com

The ratio of kinetic energy and potential energy of an electron in a Bohr orbit of a hydrogen - like series is :
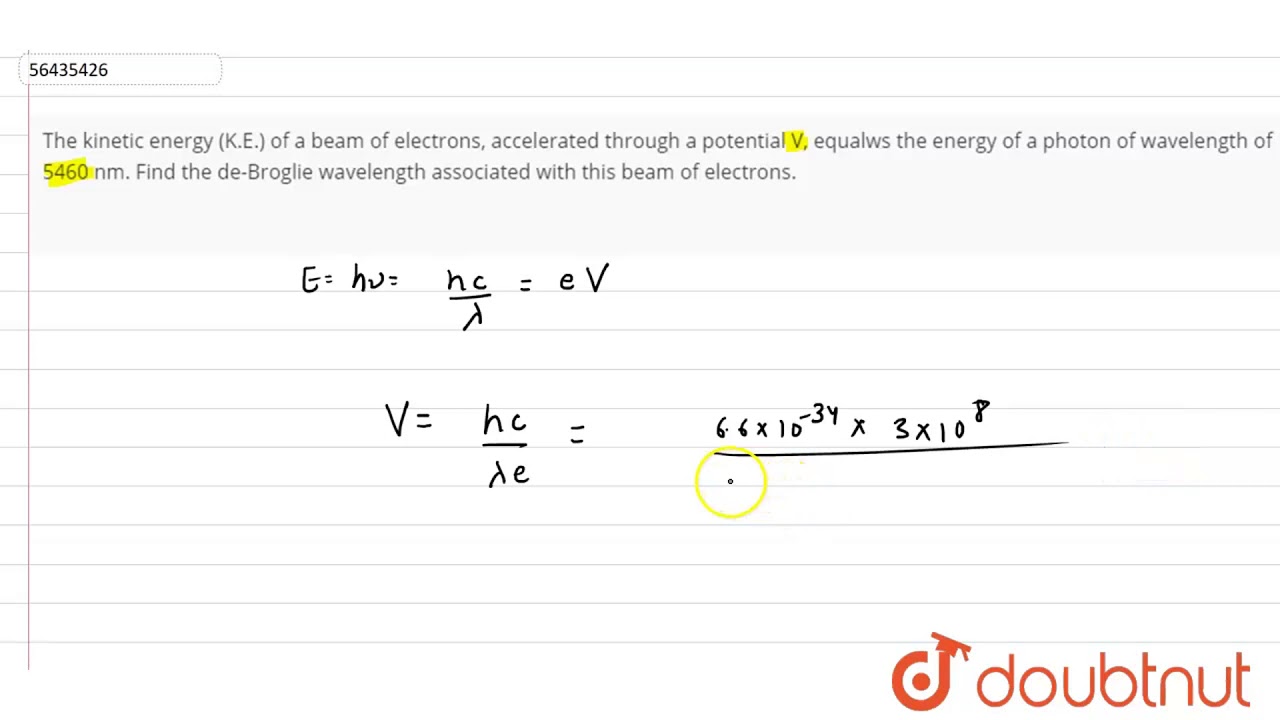
The kinetic energy (K.E.) of a beam of electrons, accelerated through a potential V, equalws - YouTube

![The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube](https://i.ytimg.com/vi/He9LjP05EHA/maxresdefault.jpg)

![Solved Problems - Engineering Physics [Book] Solved Problems - Engineering Physics [Book]](https://www.oreilly.com/api/v2/epubs/9788131775073/files/images/co4n066.png)